Pickle plus mains
Back in February 2014, it was a cold (but dry) winter’s night and we decided to warm our hearts with some science.
Our experiment was to observe the conduction of electricity through a pickle, using salt ions. We connected a pickle to the mains supply. Pickles have a strong table salt content, which means a lot of sodium. Sodium, when suitably excited by electricity, will emit light with peaks around 589nm. This is the distinctive “sodium yellow” that is familiar from street lighting.
I won’t go on about excitation energy and ionisation potential (I last studied chemistry a cool 20 years ago…) but suffice it to say that mains voltage will ionise the hell out of many things.
Caution!
I have to point out that doing this kind of thing is dangerous, especially if you don’t take strong safety measures. Mains electricity is NOT a toy; don’t go near it unless you are able to do so safely. I will not be held responsible if you hurt yourself!
Experimental setup
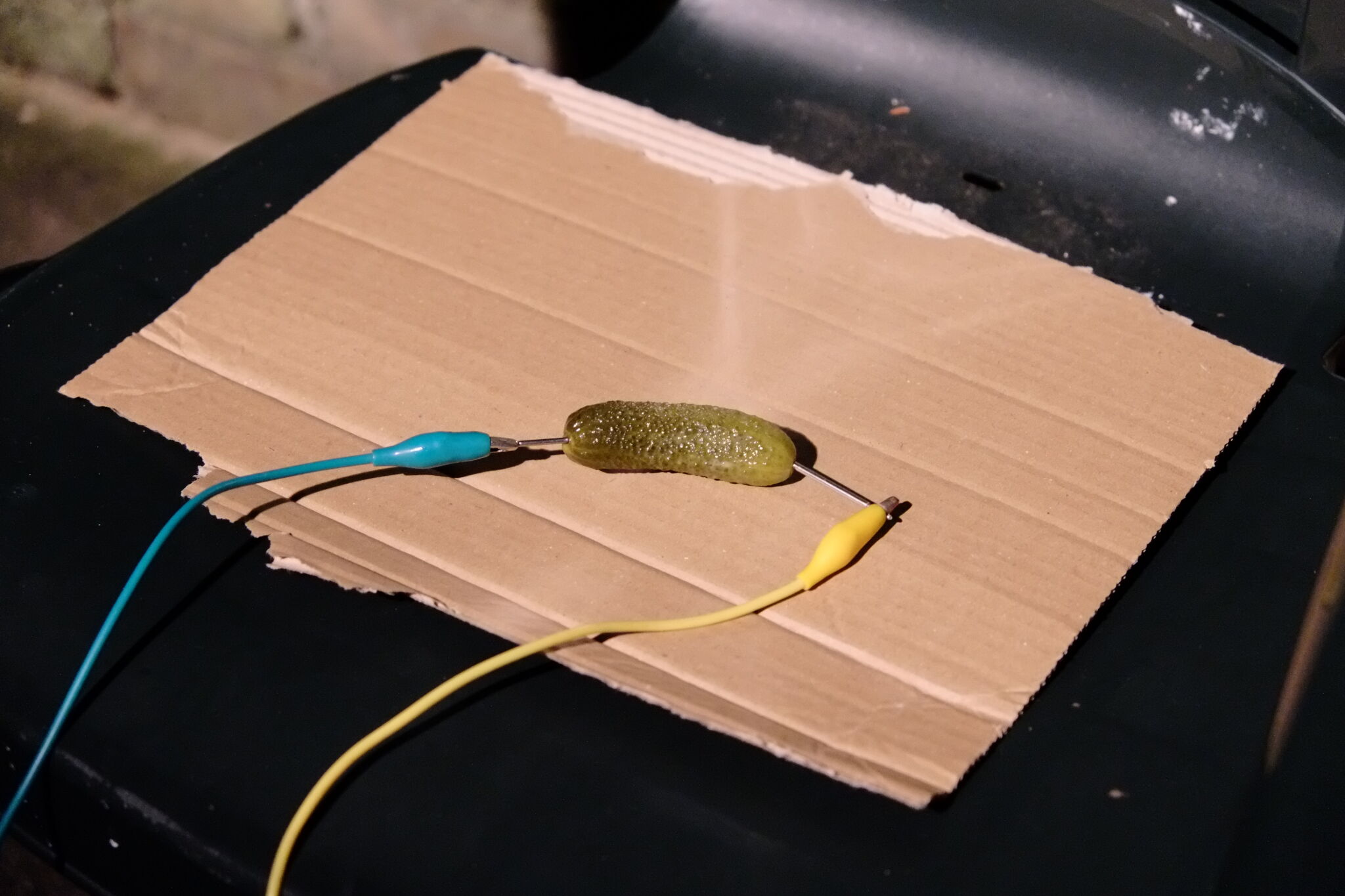
… okay, let me explain:
- We selected a dry area, away from anything conductive or flammable. We chose a concrete patio in the garden, with plenty of space.
- We placed the pickle on a garden chair with a layer of safety cardboard.
- The mains power is obviously a dangerous part. Using a long extension lead, we separated ourselves from the experiment by several metres. This allowed us to start/stop the current from a distance.
- I didn’t do this alone and ensured my experiment partner knew what to do in case of an electric shock. We didn’t rush and we always made sure to verbally cross-check that power is off when checking/re-arranging the connections.
- We inserted two iron nails into the ends of the pickle as electrodes. The electrodes were about 1cm deep in the pickle, separated by about 3cm. We connected the electrodes to an IEC mains cable using a set of crocodile clip/cables.
- We tied down the electrical cables, so they were limited in what they could touch in the case of them sparking and jumping.
We prepared for the following:
- The pickle is going to fizz & spit, and this will move the electrodes
- The electrodes might touch together – if that happens, it will be pretty bad and can send sharp hot metal flying.
- Or, they might fall or fly elsewhere, and touch something else. We made sure that if that were to happen, that something else couldn’t be a living thing. (Cat locked indoors 😿)
- Fire 💥
- We kept fire-extinguishing apparatus nearby (extra points for CO2 extinguishers for electrical fires)
- A nasty stink 🤢
- Cool pictures, using a long zoom lens so I didn’t have to go lean over it while it fried. :-)
Lighting it up

We retreated and powered it up for about two seconds. A fizzing, buzzing and lovely yellow glow resulted! With a few more tries, we got it glowing for a few seconds at a time. What would happen is that the electrodes would burn a hole that would then dry up and lose contact, needing rearrangement.
Note that the pickle is glowing only on one end, around one electrode. Given that mains is AC, there shouldn’t be a distinction between either electrode (there is no overall “anode” or “cathode”). Wikipedia says that this phenomenon is a mystery (at least, a mystery to me and the Wikipedia article author, I guess).


In some labs, a crispy and burned pickle is a delicacy amongst chemists.

Gross.